Stentdesign und FEA (simulation)
STENT-DESIGN (LASERCUT)
Unsere Stärken
- Stent Konzeption
- Stent Konstruktion (2D and 3D)
- Stent nummerisch validieren und qualifizieren mit Hilfe Finite Elemente Simulation
- Fertigungszeichnungen bereitstellen (Lasercut-drawing)


STENTVALIDIERUNG UND OPTIMIERUNG
Folgende Parameter können optimiert werden:
- Radialkraft
- Flexibilität im Gefäß
- Distale und Proximale Stent-Seite
- Crimpverhalten
- Expansionsverhalten
- Fatigue (Lebensdauer)
Folgende Internationalen Normen und Regelwerke werden in diesem Rahmen verwendet:
ISO 25539-1: Cardiovascular implants – Endovascular devices – Part 1: Endovascular prostheses
ISO 25539-2: Cardiovascular implants – Endovascular devices – Part 2: Vascular stents
ISO 25539-3: Cardiovascular implants – Endovascular devices – Part 3: Vena cava filters
ASTM F2514: Finite Element Analysis (FEA) of Metallic Vascular Stents Subjected to Uniform Radial Loading
ASTM F2477: Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents
ASTM F2942: Standard Guide for in vitro Axial, Bending, and Torsional Durability Testing of Vascular Stents
ASTM F3067: Guide for Radial Loading of Balloon Expandable and Self Expanding Vascular Stents
ASTM F2079: Elastic Recoil of Balloon-Expandable Stents
ASTM F3211: Fatigue-to-Fracture (FtF) Methodology for Cardiovascular Medical Devices.
FDA Guidance for Industry and FDA Staff: Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems. April 18, 2010
FDA Guidance for Industry and FDA Staff: Reporting of Computational Modeling Studies in Medical Device Submissions. September 21, 2016
Heart Valves:
ISO 5840-3: Cardiovascular implants – Cardiac valve prostheses – Part 3: Heart valve substitutes implanted by transcatheter techniques
ISO 5910: Cardiovascular implants and extracorporeal systems – Cardiac valve repair devices


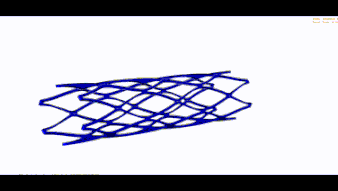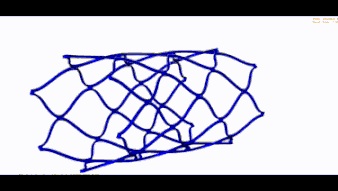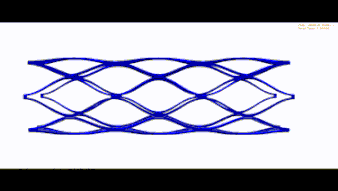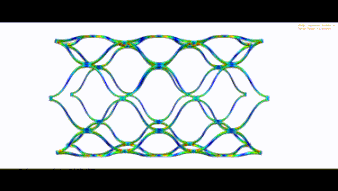

STENT FEA (BRAIDED UND LASERCUT)
Wir übernehmen den nummerischen Nachweis Ihres Stents und führen folgende Simulationen durch:
- Radialkraft (im intended used Bereich des Stents)
- Crimpen (radial comprimieren bis Katheter ID)
Wärmebehandelte Materialparameter verwenden (zB: NiTinol wärmebehandelt) !!!
Expandieren (bis zum gewünschten Durchmesser)
- Stent Fatigue ( Stent-Dauerfestigkeit im menschlischen Gefäß)
- Stent deployment (Freisetzung)
- Stent foreshortening (Stent axial komprimieren)
- Virtual Stenting (Stentimplantation)
Folgende Internationalen Normen und Regelwerke werden in diesem Rahmen verwendet:
ISO 25539-1: Cardiovascular implants – Endovascular devices – Part 1: Endovascular prostheses
ISO 25539-2: Cardiovascular implants – Endovascular devices – Part 2: Vascular stents
ISO 25539-3: Cardiovascular implants – Endovascular devices – Part 3: Vena cava filters
ASTM F2514: Finite Element Analysis (FEA) of Metallic Vascular Stents Subjected to Uniform Radial Loading
ASTM F2477: Standard Test Methods for in vitro Pulsatile Durability Testing of Vascular Stents
ASTM F2942: Standard Guide for in vitro Axial, Bending, and Torsional Durability Testing of Vascular Stents
ASTM F3067: Guide for Radial Loading of Balloon Expandable and Self Expanding Vascular Stents
ASTM F2079: Elastic Recoil of Balloon-Expandable Stents
ASTM F3211: Fatigue-to-Fracture (FtF) Methodology for Cardiovascular Medical Devices.
FDA Guidance for Industry and FDA Staff: Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems. April 18, 2010
FDA Guidance for Industry and FDA Staff: Reporting of Computational Modeling Studies in Medical Device Submissions. September 21, 2016
Heart Valves:
ISO 5840-3: Cardiovascular implants – Cardiac valve prostheses – Part 3: Heart valve substitutes implanted by transcatheter techniques
ISO 5910: Cardiovascular implants and extracorporeal systems – Cardiac valve repair devices

